Briefly Explain the Difference Between Oxidation and Reduction
Loss of oxygen 2. Gain of hydrogen 3.

Difference Between Oxidation And Reduction Compare The Difference Between Similar Terms
A Briefly explain the difference between oxidation and reduction electrochemical from MAAE 2700 at Carleton University.
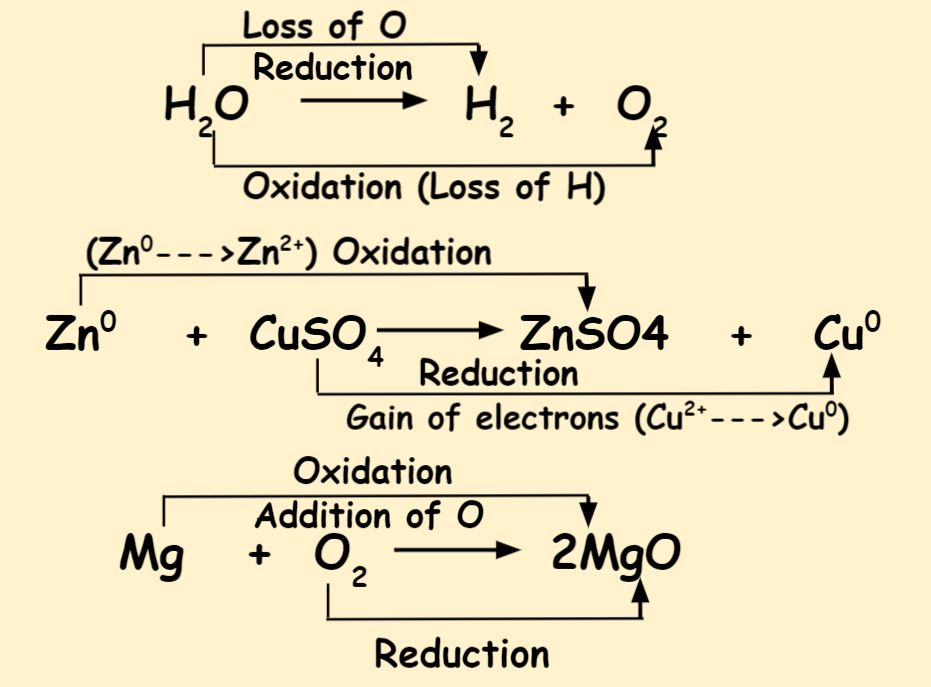
. Oxidation is addition of. Oxidation and Reduction reactions are always interlinked. This is because in reduction a material is gaining an electron while in oxidation the material is loosing the.
Oxidation is a chemical process involving the loss of electrons by a molecule atom or ion which increases the oxidation state of the chemical species. Gain of electrons Eg. 2 1 C l 2 e C l.
Oxidation involves an increase in oxidation number while reduction involves a decrease in oxidation number. Loss of electrons Eg. The main difference between oxidation and reduction is that oxidation is the increase of oxidation state whereas reduction is the decrease of oxidation state.
The key difference between oxidation and reduction is that oxidation refers to the loss of electrons while reduction refers to the gain of electrons. Reduction and oxidation both occur simultaneously. The main difference between oxidation and reduction is that oxidation occurs when a chemical compound gains oxygen or loses hydrogen.
It occurs when there is a gain of electrons. M g M g 2 2 e 3. Briefly explain the difference between oxidation and reduction electrochemical reactions oxidation is the process by which an atom gives up an electron to become a cation.
Usually the change in oxidation number is associated with a. The main difference between the reduction and oxidation process is based on gaining and loosing of electron. Oxidation is loss Reduction is gain.
Reduction is the process by which an atom an extra electron for electrons and. A redox reaction is a chemical reaction that occurs through the. Reduction is a chemical process involving the gain of electrons by a molecule atom or ion that leads to a decrease in oxidation states of the chemical species.
2 marks b Write the possible oxidation and reduction. Reduction is the process by which an atom acquires an extra electron or. 5 rows Difference between Oxidation and Reduction.
While oxidation increases the. 5 rows Oxidation is a process when an atom molecule or an ion gains Oxygen or loses one or more number. When one molecule gains an electron is termed reduction.
This is the opposite process of oxidation. QUESTION 3 a Briefly explain the difference between oxidation and reduction electrochemical reactions. Because electrons are neither created nor destroyed in a chemical reaction oxidation and.
Answer a Oxidation is the process by which an atom gives up an electron or electrons to become a cation. Loss of hydrogen 2. When one molecule loses an electron it is termed oxidation.
This means the positive charge. Main Difference Oxidation vs Reduction. Both oxidation and reductions typically occur at the same time.
Oxidation and reduction are the two half reactions of redox reactions. Gain of oxygen 1. Vit Answeraoxidation is the process by which an atom an electron for electrons to become a cation.
Difference Between Oxidation And Reduction Definition Mechanism Examples

Difference Between Oxidation And Reduction Chemistry Basics Chemistry Classroom Chemistry Lessons

10 Differences Between Oxidation And Reduction Reaction Dewwool

No comments for "Briefly Explain the Difference Between Oxidation and Reduction"
Post a Comment